ESTERS OF ORGANIC ACIDS MFCA (BUTYRIC, CAPRYLIC, CAPRIC, PROPIONIC LAURIC) SHORT AND MEDIUM CHAIN MCFA (MEDIUM CHAIN FATTY ACIDS) ESTERIFIED WITH GLYCEROL IN ANIMAL NUTRITION
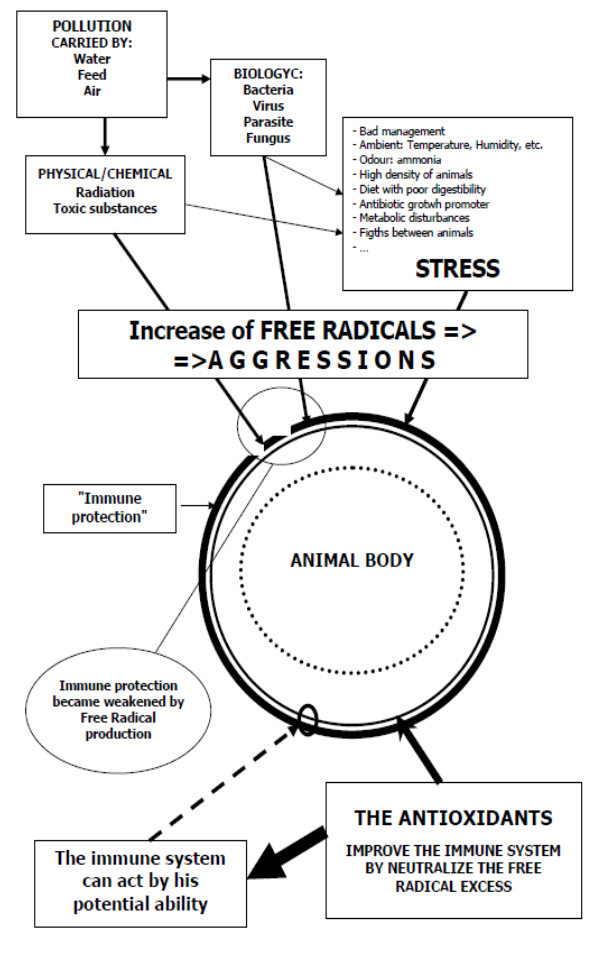
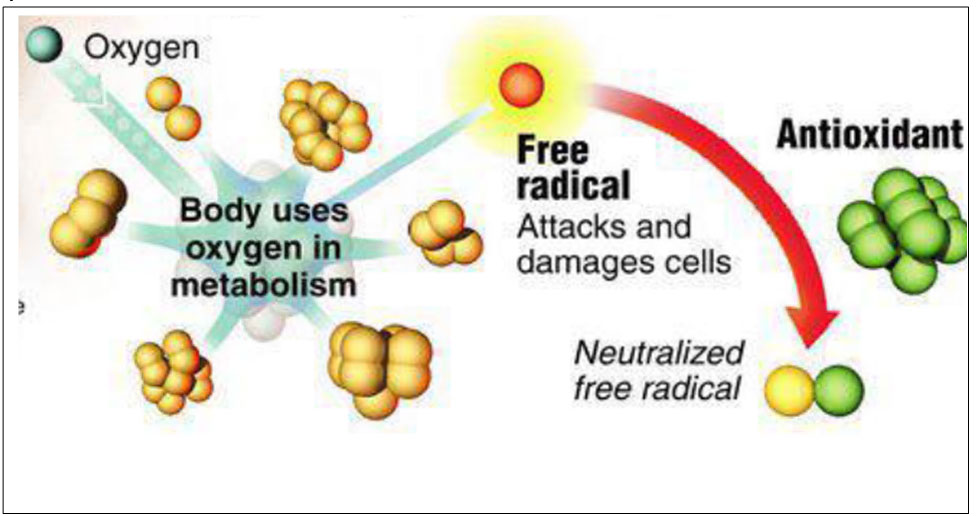
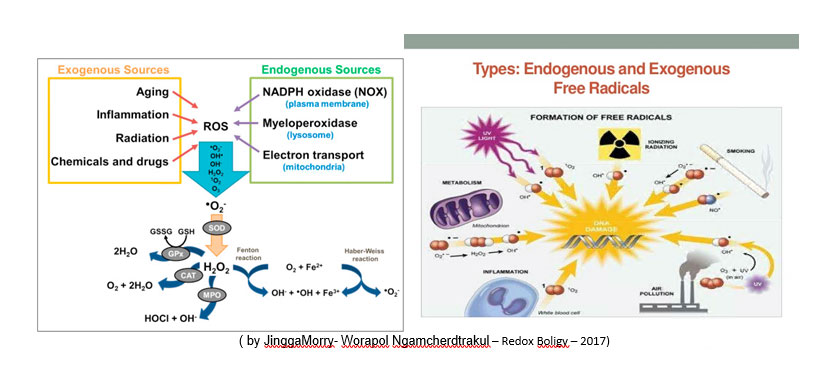
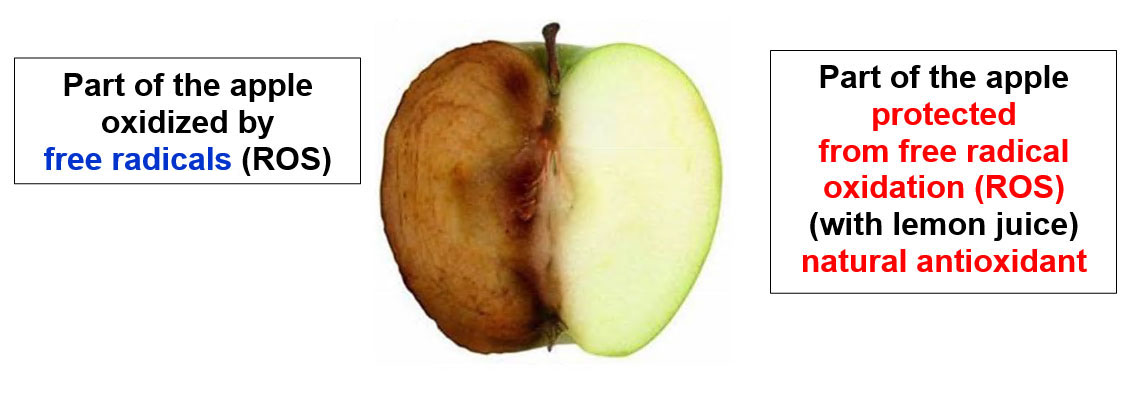
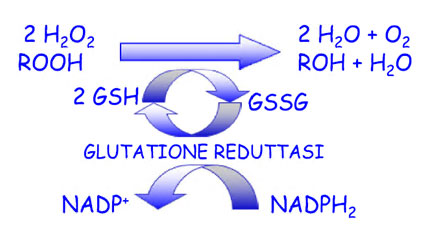
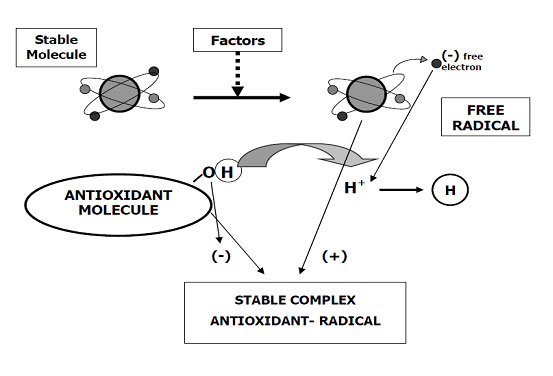
The zootechnical challenge of the next few years that will affect health and productivity in the industrial livestock sector will be to be able to control the oxidative stress of farmed animals by associating at the same time alternative products to the use of antibiotics (antibiotic-free) in order to contain the main problems of the digestive system through the use of natural products and the use of phyto-derivatives with a degree of purity and intracellular concentration obtained “mechanically” without the use of “chemical contaminants” such as solvents with repercussions on animal health , of the environment and of people by combining polyphenols, bioflavonoids, medicinal plant products with anti-inflammatory and medicinal action, MCFA (medium and short chain fatty acids esterified with glycerol) associated with controllers and stimulators of the digestive microbiome such as -pre-postbiotics ( Dr. Giulio Gabaldo – 2018)
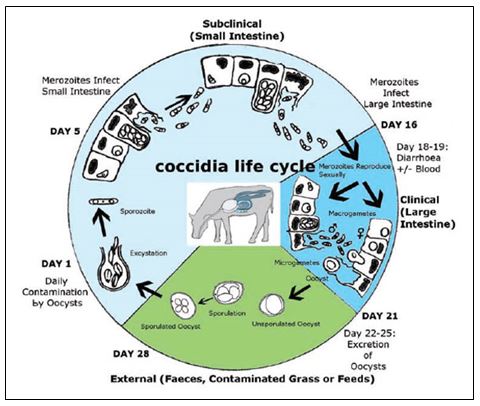
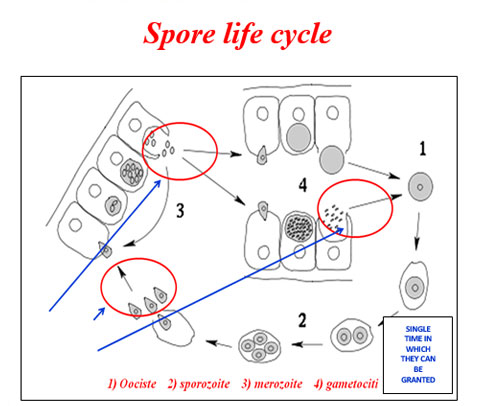
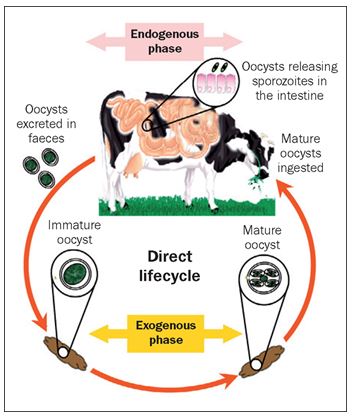
BACTERICIDE MECHANISM OF ACTION OF MCFA
(by Ward Dean, MD and Jim English – 2013)
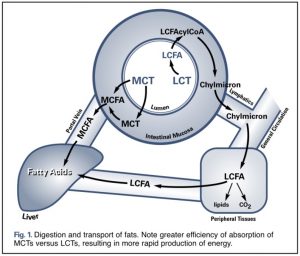
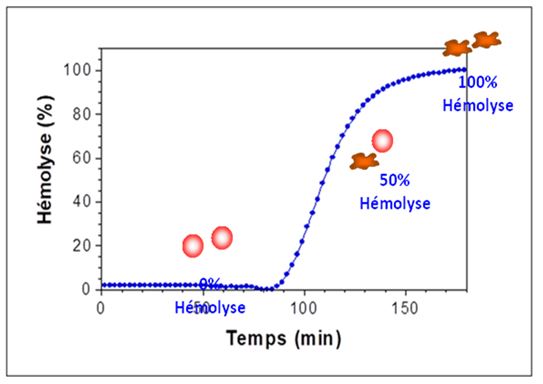
The antibacterial action of the acid depends on the variation of the pH in general. At low pH values they increase the concentration of MCFA (<C4) need to be in the undissociated state to enter the bacterial cell. After entering the cell, the high intracellular pH value leads to their dissociation. The lowering of the internal pH then disrupts the normal metabolism (Ricke, 2003). On the other hand, when the pH rises, it totally loses this ability. Consequently, the antibacterial action is expressed only if the MCFA remains undissociated, that is, in an environment <4.5. When the pH rises ± 7, (second part of the intestine) the MCFA lose this ability and to enter they need the action of the aguaporins which are intrinsic proteins found in the cell membrane wall and which allow the flow of water. in bidirectional sense. Two families of aquaporins have been identified:
a) Specific aquaporins: they only allow the transport of water. The channel is in fact made up exclusively of amino acids, which only bind water molecules while other ions and molecules do not pass through this channel.
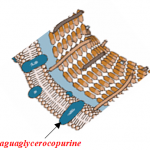
b) Aquaglyceroporins: these also allow the passage of water, but unlike the previous ones, they allow the passage of glycerol and other neutral molecules connected to it.
CHEMICAL COMPOSITION OF THE ESTERS OF MCFA ESTERIFIEDWITH GLYCEROL (by SILO – SpA- FI)
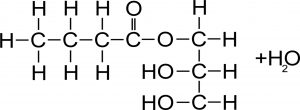
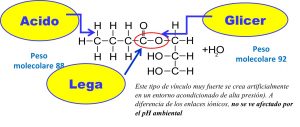
1) Non-esterified MCFA can have an anti-inflammatory and anti-bacterial action only in the 1st part of the intestine (small intestine 2) where the pH is still low (± 4). After that, with the balancing of the pH by the bile to pH ± 7, they become inactive. Increasing the dosages causes loss of appetite and a corrosive action on the intestinal wall.
2) MCFA esterified with glycerol, having a stable covalent bond, can “go down” in the lower part of the intestine (fat n ° 3 and 4) in an unaltered form and by means of the aquaglyceroporin mechanism they can “hook” the pathogenic germs (using the glycerol as a transport carrier)
CONCLUSIONS: through the use of esterifieds with MCFA glycerol an effective antibacterial action is obtained without the use of antibiotics ( antibiotic free) on the main intestinal pathogenic families of swine, poultry, horses, etc. (Coli, Clostridia, Salmonella, Brachispira, Rhodococcus, etc.) obtaining at the same time an anti-inflammatory action without harming the positive lactic population of the intestine