What are oxidizing substances or ROS (Reactive Oxygen Species)?
In chemistry it is said that a chemical element undergoes oxidation when it undergoes an electron subtraction, which translates into an increase in its oxidation number. This subtraction of electrons can take place by another element, which thus undergoes the complementary reduction process. Most oxidation reactions involve the development of energy in the form of heat and electricity. Substances that have the ability to oxidize other substances are known as oxidizing agents or ROS.
They subtract electrons from other substances and in practice accept electrons. Oxidizers are generally chemical substances that possess elements with a high number of oxidation, for example hydrogen peroxide, permanganate or highly electronegative substances such as oxygen (eg: air), flower, chlorine (eg: sea salt) or bromine, capable of removing one or more electrons from other substances.
Oxidation
Simple classic examples:  Piece of oxidized metal (corroded) – Corrosion
Piece of oxidized metal (corroded) – Corrosion
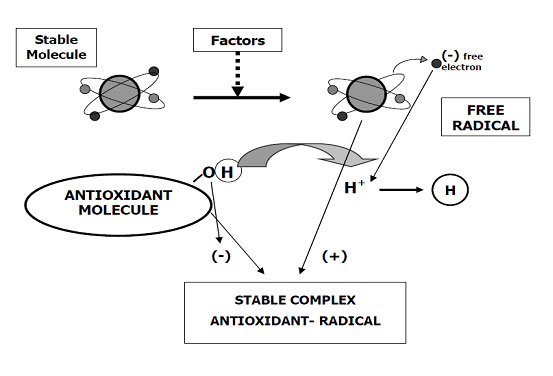
Oxidation is a chemical reaction that transfers electrons from a substance to an oxidizer.
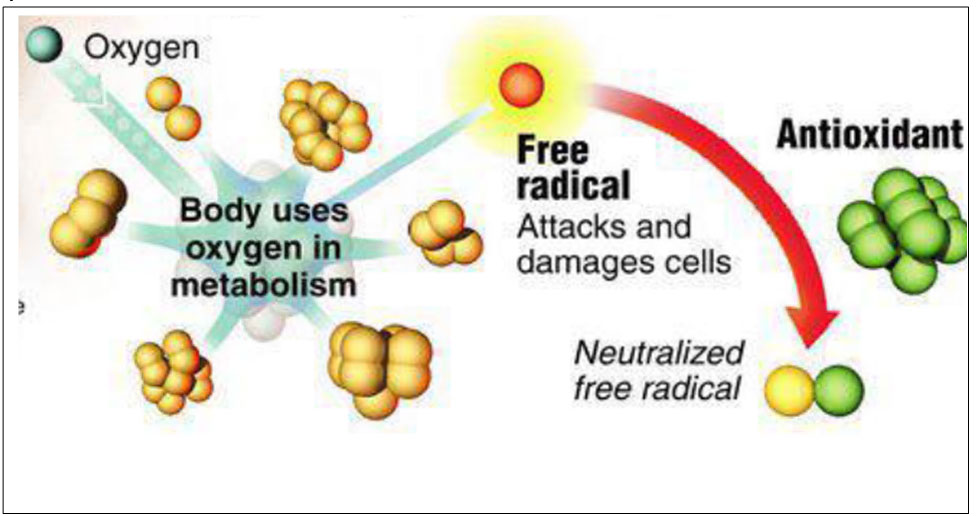
Oxidation reactions produce free radicals or ROS, which are responsible for initiating a chain reaction that damages cells. Antioxidants terminate these chain reactions by intervening on intermediate radicals and inhibiting other oxidation reactions by oxidizing themselves.
Oxidative stress
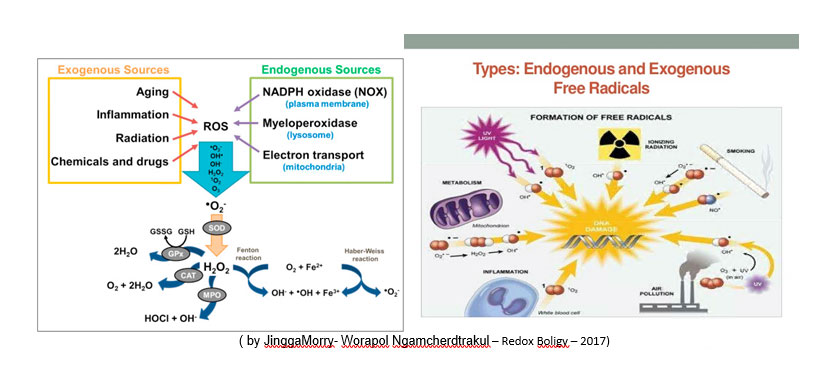
Oxidative stress is a pathological condition caused by the breakdown of physiological equilibrium, in a living organism (vegetable, animal or human), between the production and elimination, by antioxidant defense systems from oxidizing substances.
All life forms maintain an “antioxidant reducing environment” (antioxidant stock) within their cells. In the REDOX cellular environment (with the term redox or redox from the English REDduction, reduction and OXidation, oxidation) all those chemical reactions take place in which the oxidation number of the atoms changes (ie all the reactions in which there is an exchange of electrons from one chemical structure to another) it is preserved by enzymes that maintain the reduced state through a constant input of metabolic energy.
Possible alterations of the normal REDOX state can have toxic effects for the production of peroxides and free radicals that damage all the components of the cell, including proteins, fats and DNA intervening negatively in the systems of self-defense (immunodepression) and in the health of the organis.
Cellular oxidative stress
Oxidative stress, on the part of free radicals and how, these oxidative processes, can cause significant oxidation at the level of the cell membrane and destroy DNA. Today it is possible to evaluate them by means of tests that help us assess the state of health of the organism , the inflammatory state and the onset of some diseases (eg: in humans pathologies such as diabetes, Alzheimer’s and cardiovascular diseases, but also in animals as in pigs with the onset of “very aggressive viral and bacterial forms that are not very sensitive to normal drugs, such as PRRS, etc … or even, not less important, lack of productive and qualitative performances).
Today it is possible to measure both the production of free radicals and the body’s ability to react to oxidative stress through the antioxidant barrier that includes both endogenous antioxidants (complex enzyme systems) and exogenous ones (ie those that are taken through nutrition), and also antioxidant power of a particular functional food (KRL test on red blood cells).
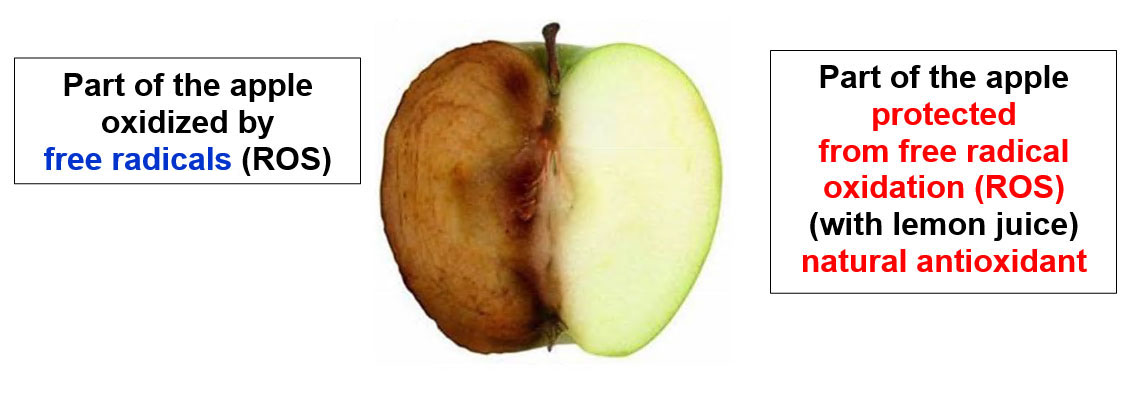
Classic example “on the apple” of damage from free radicals at the cellular level
Definition of antioxidants
Antioxidants are natural and non-natural chemical substances (molecules, ions, radicals) or physical agents that slow down or prevent the oxidation of other substances as a result. The antioxidants are chemically defined of involve as reducing agents (such as thiols and polyphenols) as the chemicals involved in to reactions in thr oxide-reducing. Although oxidation reactions are vital for life, they can be just as harmful; therefore, both plants and animals maintain multiple types of antioxidants as complex self-defense systems.
Antioxidants can be ….
- Primary:
1) When they prevent the production of “species” of radicals
2) When “grappling ” on the transition metals - Secondary: when they react with the newly formed radicals and convert them into non-reactive forms by interrupting the chain reaction and therefore can be:
- Endogenus: quando sono sintetizzati dall’organismo stesso( enzimatici cellulari) ed a seconda della loro azione posso essere
a) 1) Enzyme type cellular, as:
– SOD (superoxide dismutase), catalase and glutathio-peroxidase (it is the main cellular antioxidant that maintains low O2 level and works in conjunction with Catalase and Glutathione Peroxidase (GSH-Px —-> Vit E + Se) it is the main “detoxifier” of the cells: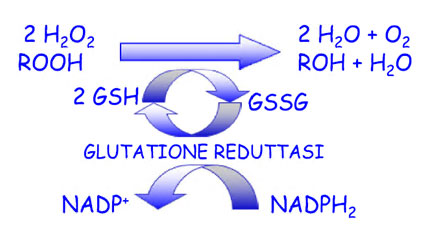
– 2. The Catalase (CAT): 2 H2 O2 ——> 2 H2O + O2
b) Type Protein as SH and metallic sequestering agents (Fe, Cu,) - Exsogenus:
– Vitaminics: Vitamin C and Vitamin E and Carotenoids (as provitamin A)
– Polyphenols and Bio-flavonoids
How do antioxidants work?
The oxidation process is a chemical reaction that transfers electrons from a substance to an oxidizer